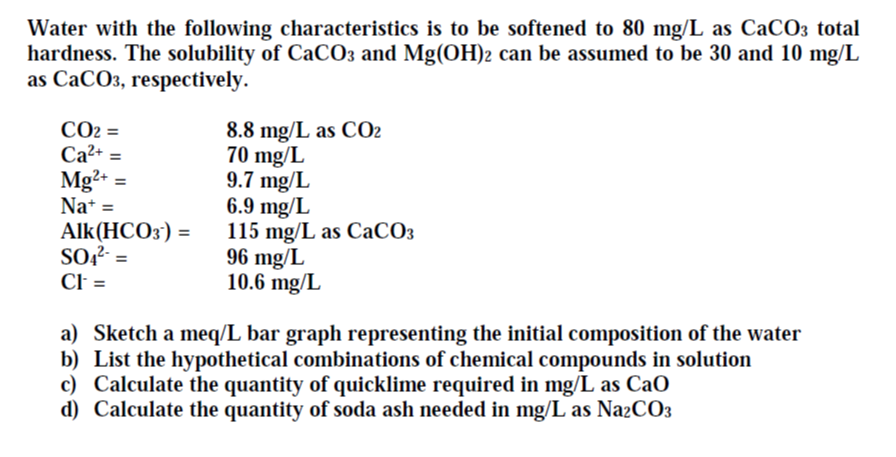
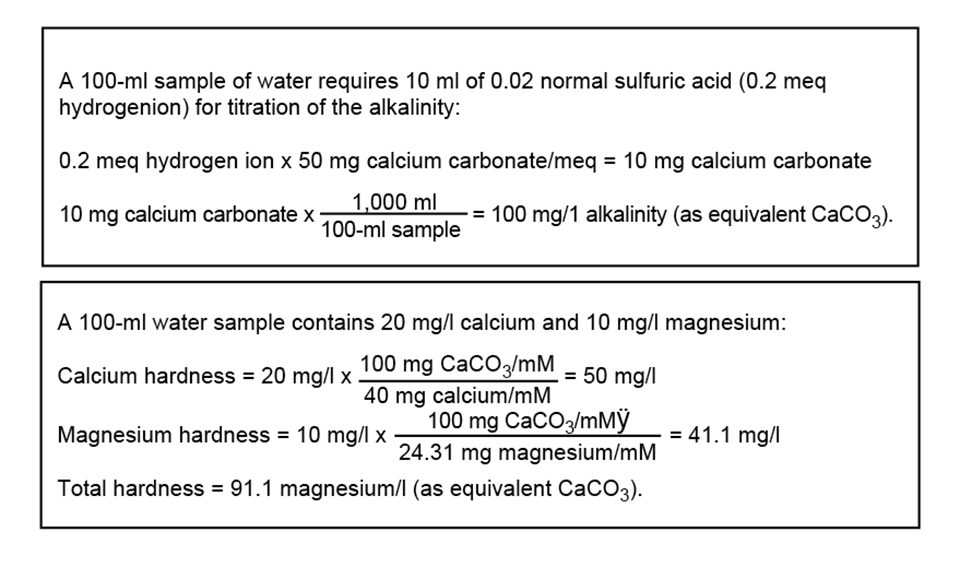
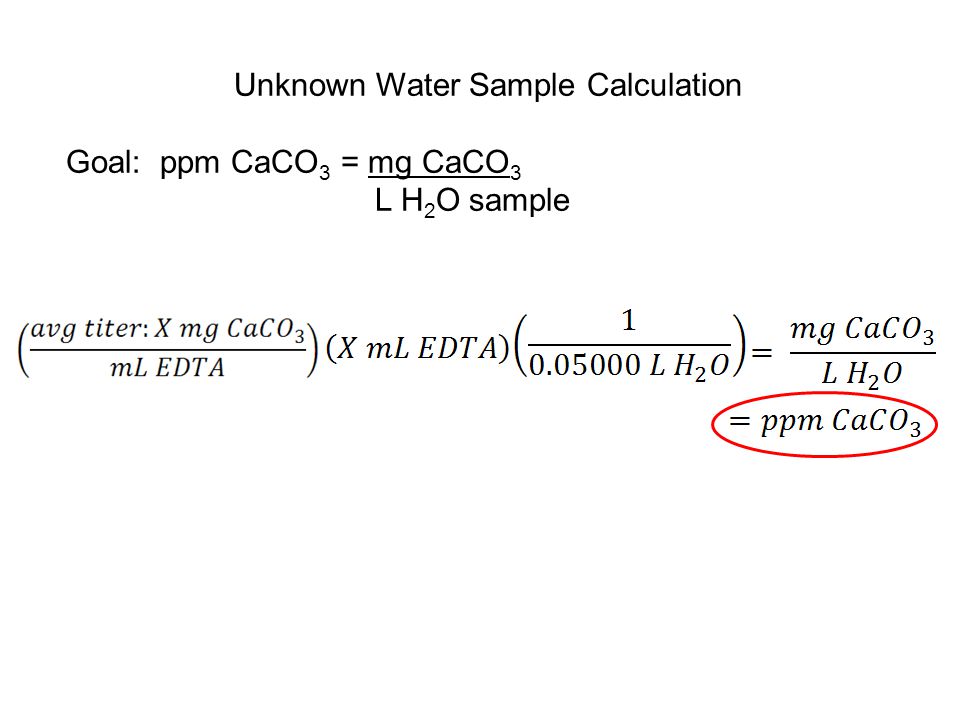
Hardness of Water 1st Step: The calcium ion coordinates with the indicator (Eriochrome Black T). H2In- + Ca2+ ↔ CaIn- + 2H1+ 2nd Step: - ppt video online download

a water sample contains 204 mg of caso4 per liter calculate the hardness in terms of caco3 equivalent - Brainly.in

SOLVED: total hardness, carbonate hardness, and noncarbonate hardness Calculate the alkalinity, for the following water in mg/L as CaCO; Anions mgL Cations mgl HCOs 135 Ca?- 94 28 SOX 134 Mg Fe2+

SOLVED: Problems Calculate total hardness, carbonate hardness, and non-carbonate hardness of the samples What is the degree ofhardness of the sample (sof, moderate; hard, very hard)? Why might we necd to know

SOLVED: Problems Calculate total hardness, carbonate hardness, and non-carbonate hardness of the samples What is the degree ofhardness of the sample (sof, moderate; hard, very hard)? Why might we necd to know
![The hardness of a water sample (in terms of equivalents of CaCO3) containing 10^-3 M CaSO4 is:[Molar mass of CaSO4 = 136 g mol^-1] . The hardness of a water sample (in terms of equivalents of CaCO3) containing 10^-3 M CaSO4 is:[Molar mass of CaSO4 = 136 g mol^-1] .](https://dwes9vv9u0550.cloudfront.net/images/8020054/a6e110e8-cb36-4333-8440-cae1f5196351.jpg)
The hardness of a water sample (in terms of equivalents of CaCO3) containing 10^-3 M CaSO4 is:[Molar mass of CaSO4 = 136 g mol^-1] .

PDF) 21 Water Softening CALCULATING CALCIUM HARDNESS AS CACO 3 | Shariful Haque Robin - Academia.edu

One litre of a sample of hard water contains 55.5 mg of CaCl2 and 4.75 mg of MgCl2 . The total hardness in terms of ppm of CaCO3 is :

Hardness Objective n to understand the chemical basis of water hardness, how it originates, and ways it can affect water distribution systems. n to know. - ppt download