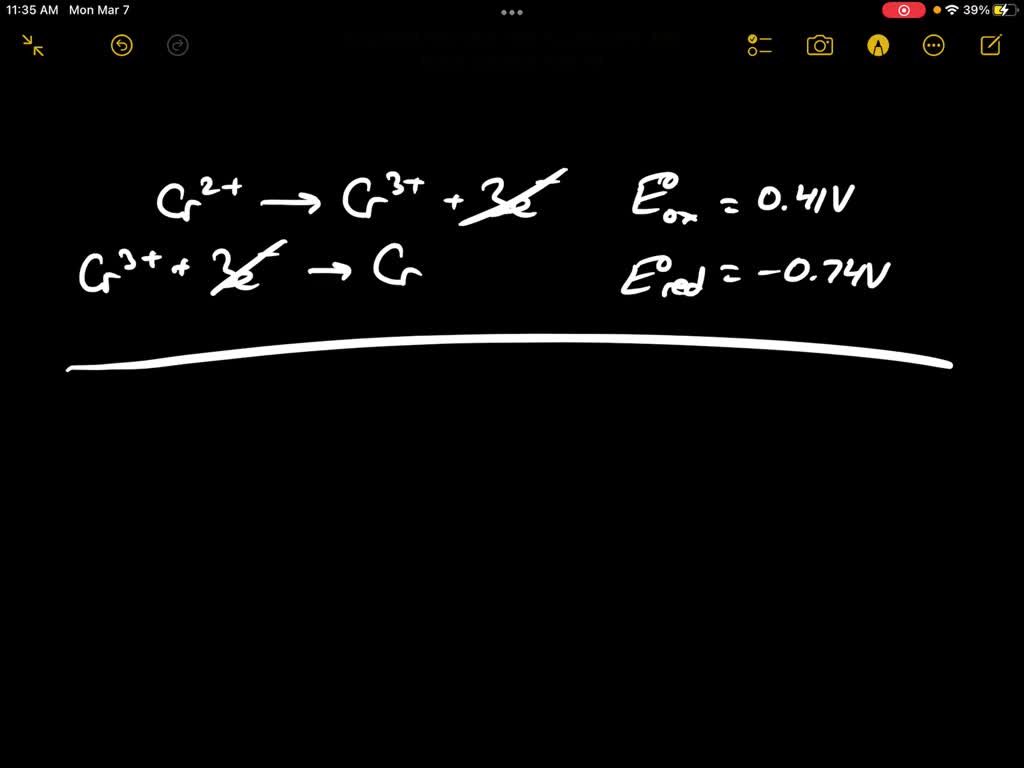
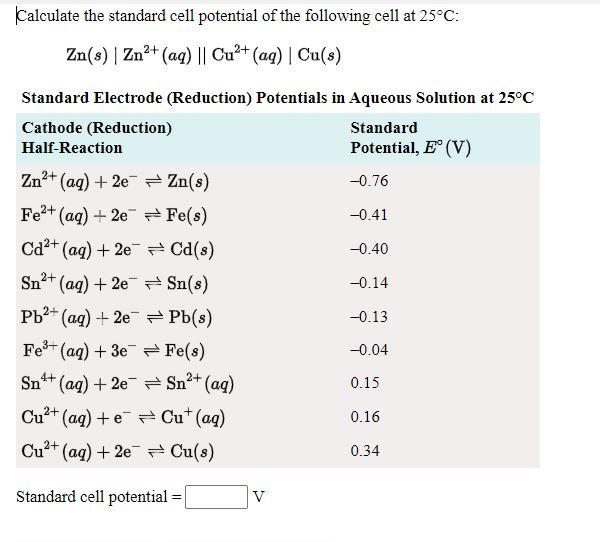
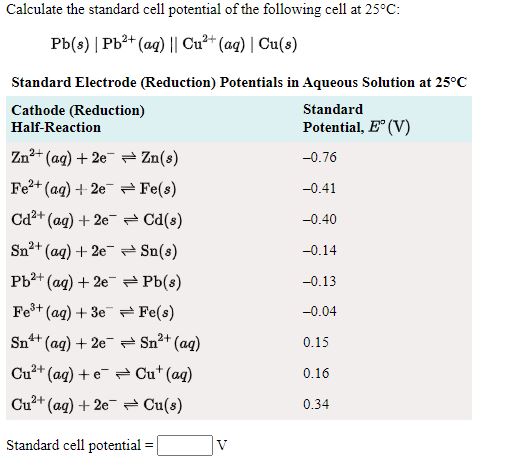
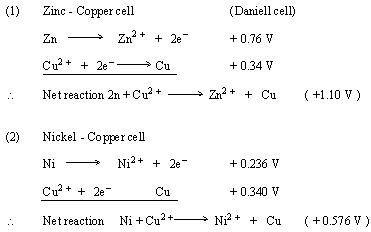
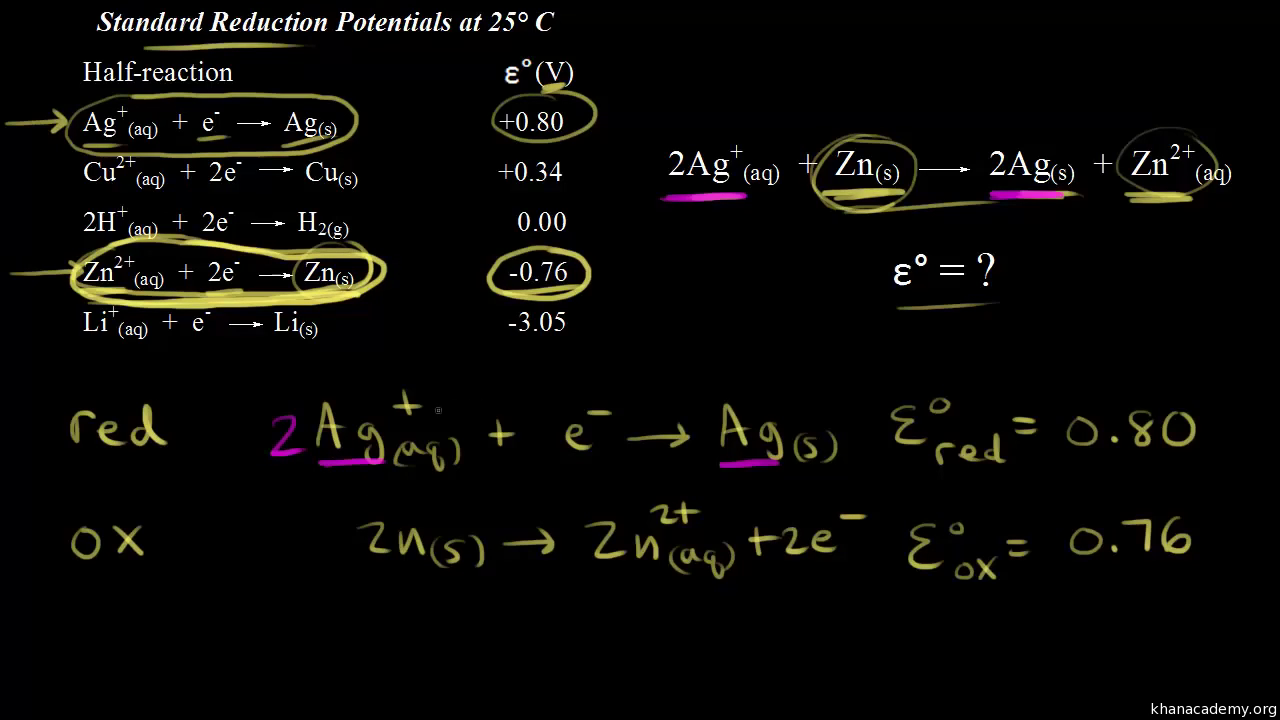
Calculate the standard cell potentials of galvanic cell in which the following reactions take place:(i) 2Cr(s) + 3Cd^2 + (aq) → 2Cr^3 + (aq) + 3Cd (ii) Fe^2 + (aq) + Ag^+ (
How would you determine the standard electrode potential of Mg^2+/Mg? - Sarthaks eConnect | Largest Online Education Community
![SOLVED: Calculate the EMF of cells A through F at standard states (E"cel) and non-standard states (Eccll) using Nernst equation. Fill in Table-] with your answers b The Standard Electrode Potential of SOLVED: Calculate the EMF of cells A through F at standard states (E"cel) and non-standard states (Eccll) using Nernst equation. Fill in Table-] with your answers b The Standard Electrode Potential of](https://cdn.numerade.com/ask_images/bd798f86fb6f4631925af95715b183c5.jpg)
SOLVED: Calculate the EMF of cells A through F at standard states (E"cel) and non-standard states (Eccll) using Nernst equation. Fill in Table-] with your answers b The Standard Electrode Potential of

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa

Calculate the standard electrode potential of the Ni^(2+)//Ni electrode , if the cell potential potential of the cell, Ni//N^(2+)(0.01 M)//Cu is 0.59" V ". "Given" E(Cu^(2+)//Cu)^(@)=+0.34 " V "
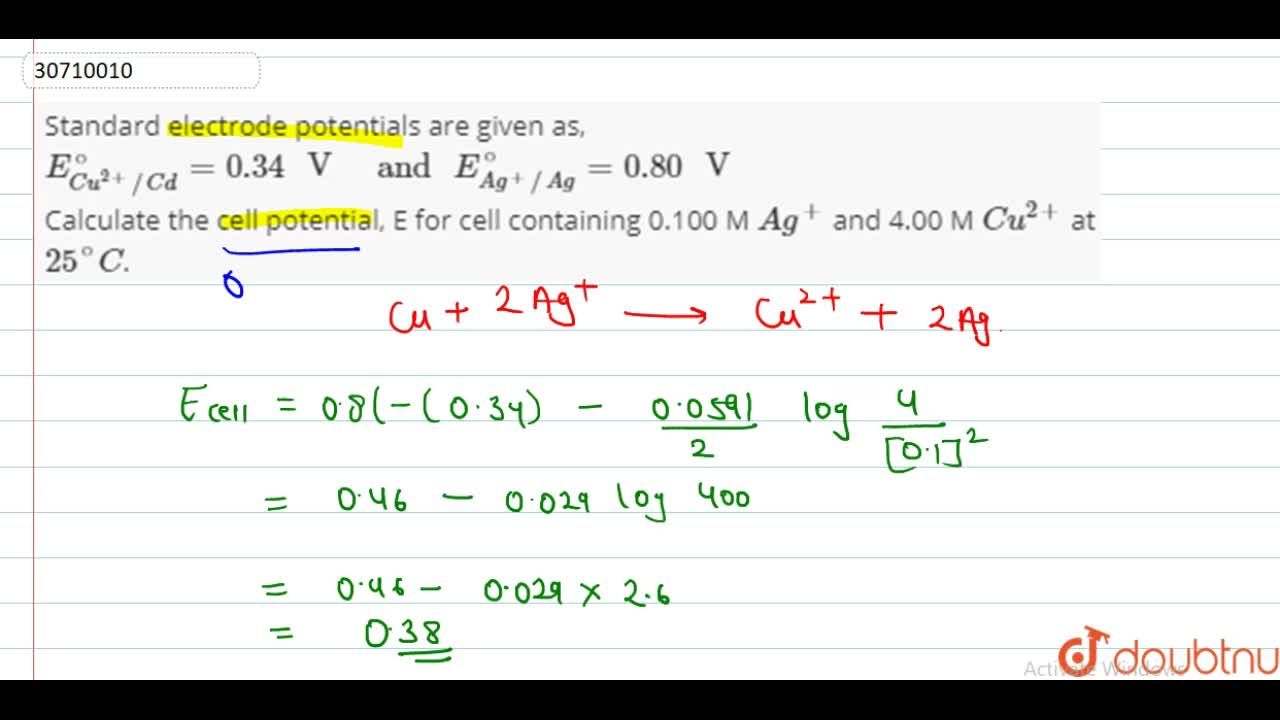
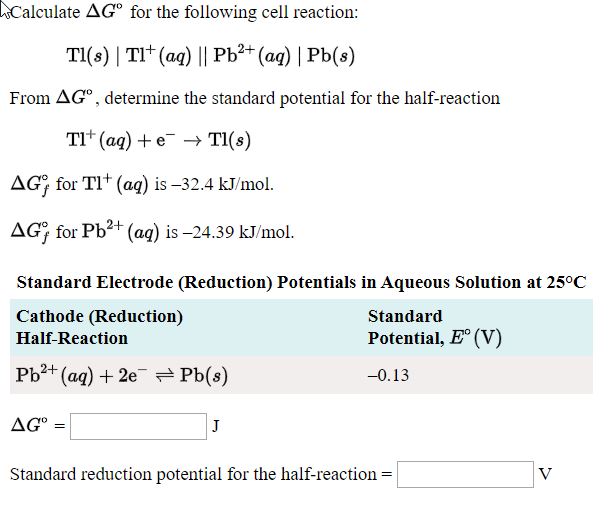
Standard electrode potentials are given as, E(Cu^(2+)//Cd)^(@)=0.34" V " " and " E(Ag^(+)//Ag)^(@)=0.80" V " Calculate the cell potential, E for cell containing 0.100 M Ag^(+) and 4.00 M Cu^(2+) at 25^(@)C.

Calculate the standard electrode potential of the Ni^(2+)//Ni electrode , if the cell potential ... - YouTube